difference between gravimetric and volumetric method of analysis|how to calculate gravimetric factor : Brand manufacturer Gravimetric Analysis refers to a quantitative method in which the amount of an analyte is determined based on its mass. On the other hand, Volumetric Analysis involves determining the quantity of an analyte by . The form of government is a democratic federative republic, with a presidential system. The president is both head of state and head of. Ver mais
{plog:ftitle_list}
Resultado da Download Nautilus 20. O download começou! Instale o APK e curta o game mais imersivo e quente do Brasil 🥵. Não começou?
While both methods aim to determine the amount of a particular substance, they differ in their principles, procedures, and applications. In this article, we will explore the attributes of gravimetric analysis and volumetric analysis, highlighting their similarities and differences.
Gravimetric and Volumetric Analysis are common methods used in analytical chemistry. They provide mechanism for determining the amounts of substances present in a .
Gravimetric Analysis refers to a quantitative method in which the amount of an analyte is determined based on its mass. On the other hand, Volumetric Analysis involves determining the quantity of an analyte by .(Volumetric and Gravimetric Analysis) Practical skills – Quantitative Analysis. Principles of Volumetric Analysis. Volumetric analysis is a method to determine the amount of. sample. A .The concentration is then calculated based on the mass of the precipitate and the stoichiometry of the reaction. Volumetric analysis, on the other hand, is a method of quantitative analysis . Based on the separation of analyte, gravimetric analysis is of the following 4 types: Precipitation Gravimetry: The separation of one or more components of a solution by incorporating them into a solid is accomplished .
volumetric vs gravimetric feeder
Later, as you read through the descriptions of specific gravimetric methods, this survey will help you focus on their similarities instead of their differences. It is easier to understand a new analytical method when you can .
Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you . Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . Electro gravimetric method is employed to separate the ions of a substance, often a .
Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. .The bubbling was due to the production of CO 2.. The test of vinegar with potassium carbonate is one type of quantitative analysis —the determination of the amount or concentration of a substance in a sample. In the analysis of .
volumetric and gravimetric analysis
For pure component measurements the simplest method is to have a large volume of gas connected via a valve to the chamber where the sample is present. This is effectively a combined volumetric and gravimetric experiment, with the only difference being the measured quantity, i.e. adsorbed mass vs external pressure.
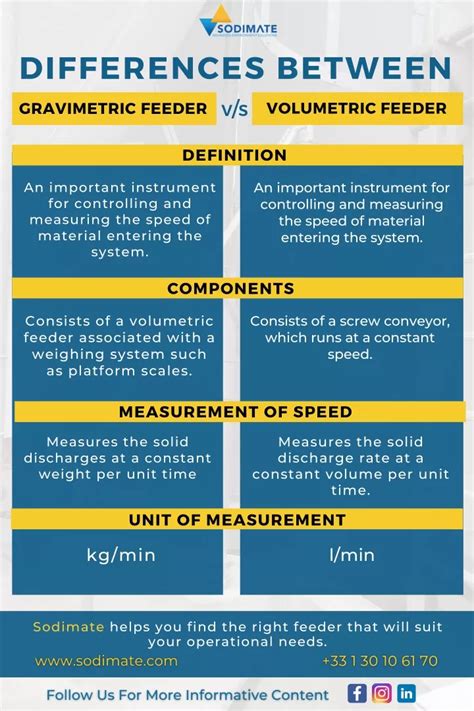
Gravimetric analysis is a quantitative method used in chemistry to determine the amount of a substance by measuring its mass. Learn types, procedure, differences etc. . To know more about its procedure, types, advantages and disadvantages, and the difference between gravimetric and volumetric analysis, . Volumetric method of analysis or sometimes referred to as titrimetric method of analysis is a quantitative method of analysis that is based upon the measurement of volume. . we get a titration error, the difference between the end point and the equivalence point, which leads to overtitration. The end point is then the point where sufficient . What’s the difference between Gravimetric Feeders and Volumetric Feeders? Feeders are auxiliary equipment that dispense a measured amount of an ingredient—a resin, a colorant, or an additive—into resin entering a processing machine. The term “volumetric” refers to one way that a feeder measures and dispenses an ingredient. This difference highlights the contrast between the two methods: gravimetric focuses on mass changes, and volumetric focuses on volume changes. 13 The accuracy of gravimetric analysis often depends on the purity of the precipitate and the completeness of the separation, while the accuracy of volumetric analysis is influenced by the precision of .
how to calculate gravimetric factor
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as . Gravimetric Analysis refers to a quantitative method in which the amount of an analyte is determined based on its mass. On the other hand, Volumetric Analysis involves determining the quantity of an analyte by measuring its volume.
Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) . The steps involved in a gravimetric analysis differ slightly between the methods used. However, in all methods, something is weighed, and the mass of an analyte is determined.
Application of Hygroscopic Gravimetric Analysis Method in Isopiestic Measurements. . In the case of unequal variances between the gravimetric and volumetric results datasets, determined by the F test, a Welch’s t test was used for comparison of the mean results. . The mean assay results showed no statistically significant differences .
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) .Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate .The first method of Volumetric Analysis was devised and found by the French chemist Jean-Baptiste-Andre-Dumas; as he was trying to determine the proportion of nitrogen combined with other elements in organic compounds. .
Gravimetric analysis is an uncomplicated, easy-to-follow laboratory method that helps in the quantitative determination of chemical compounds. It is based on measuring the amount of a chemical compound depending upon its loss in mass. How is gravimetric analysis different from a volumetric analysis method such as titration? Classical Methods of Analysis; Modern Instrumental Methods of Analysis; Analytical chemistry has a long history. On the bookshelf of my office, for example, there is a copy of the first American edition of Fresenius's A System of Instruction in Quantitative Chemical Analysis, which was published by John Wiley & Sons in 1886.Nearby are many newer texts, . Gravimetric analysis and volumetric analysis are both analytical techniques used in chemistry to determine the quantity or concentration of a substance in a sample. However, they differ in their methods and principles. Step 2/3 1. Gravimetric analysis: - Gravimetric analysis is based on the measurement of the mass of a substance.Difference between Gravimetric and Volumetric analysis: Practice Problems: Frequently Asked Questions -FAQs: Gravimetric Analysis: Gravimetric analysis is one of the commonly used quantitative methods in analytical chemistry, the other being the volumetric method. In gravimetric analysis, the amount of an ion present in an analyte is estimated .
how to calculate gravimetric analysis

In this video I have discussed difference between Gravimetric Analysis and Volumetric Analysis.#gravimetric Analysis #volumetric Analysis #quantitative.What is the difference between gravimetric and volumetric analysis? Gravimetric analysis is based on the determination of mass (atomic mass) and density of the analyte solution while volumetric analysis is used to find the volume and concentration of the analyte solution with the help of titrant solution.
The major difference between gravimetric analysis and volumetric analysis is that gravimetric analysis quantifies the mass of the analyte, whereas volumetric analysis determines the volume of the analyte. . Because the solvent temperature can be ignored, hence the gravimetric process is more accurate than the volumetric method. Solvent weight .
In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate potentially can serve as a gravimetric method. Most precipitation gravimetric methods were developed in the nineteenth century, or earlier, often for the analysis of ores .Q-5: What is the distinction between gravimetric and volumetric analysis? Answer: Both gravimetric and volumetric analysis are quantitative methods for calculating the amount of sample in a solution or the purity of a compound; gravity analysis determines the mass of the analyte, while volumetric analysis determines the volume of the analyte.Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. . The difference between the filter's original mass and final mass gives the mass of suspended solids. It is a direct analysis because the analyte itself is the object being .
gravimetric vs volumetric water content
gravimetric vs volumetric energy density
WEBVocê pode assistir "Euphoria - Temporada 2" no HBO Max, NOW em Stream legalmente. Sinopse The lines between fantasy and reality begin to blur as Kat contemplates ending .
difference between gravimetric and volumetric method of analysis|how to calculate gravimetric factor